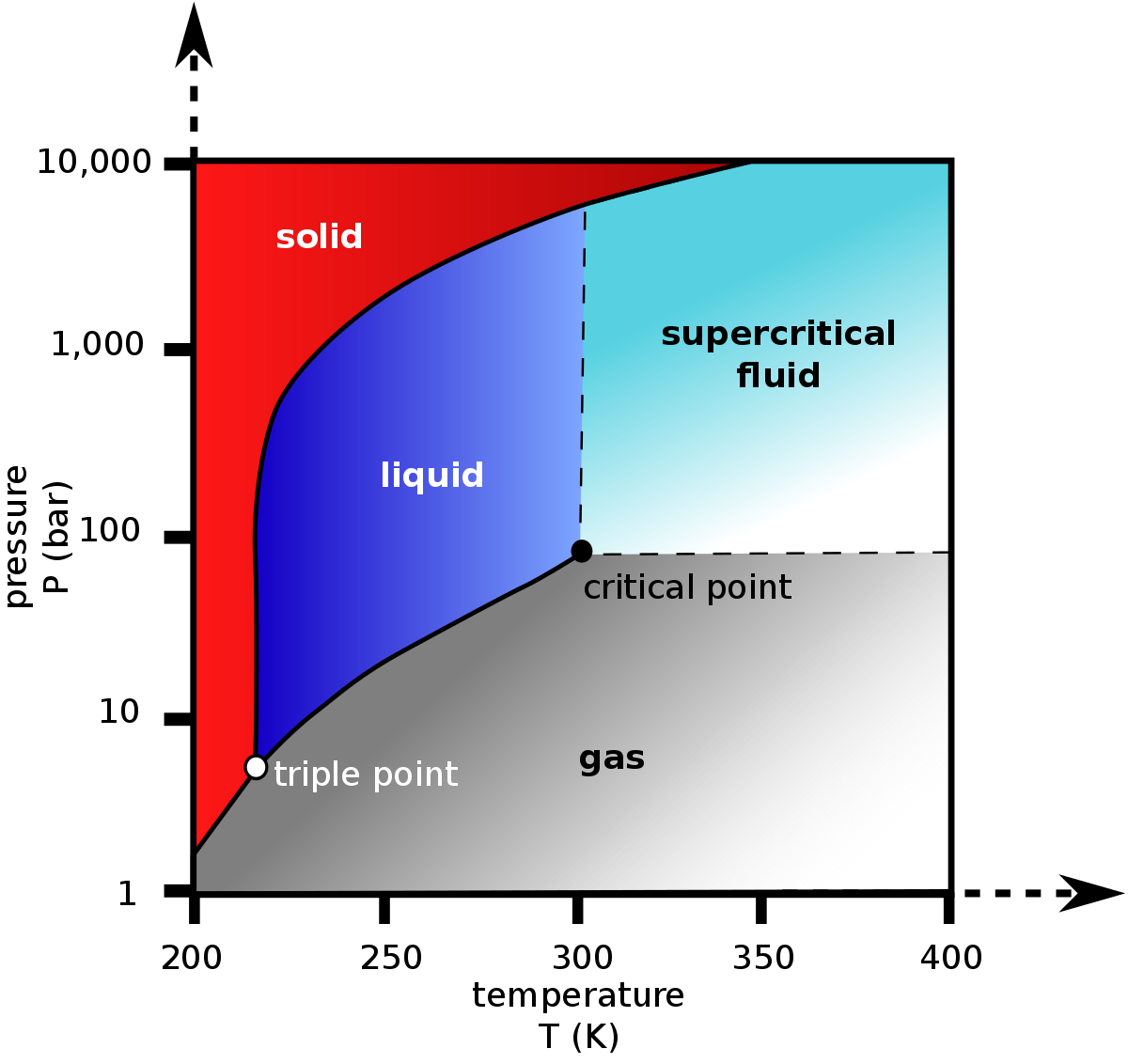
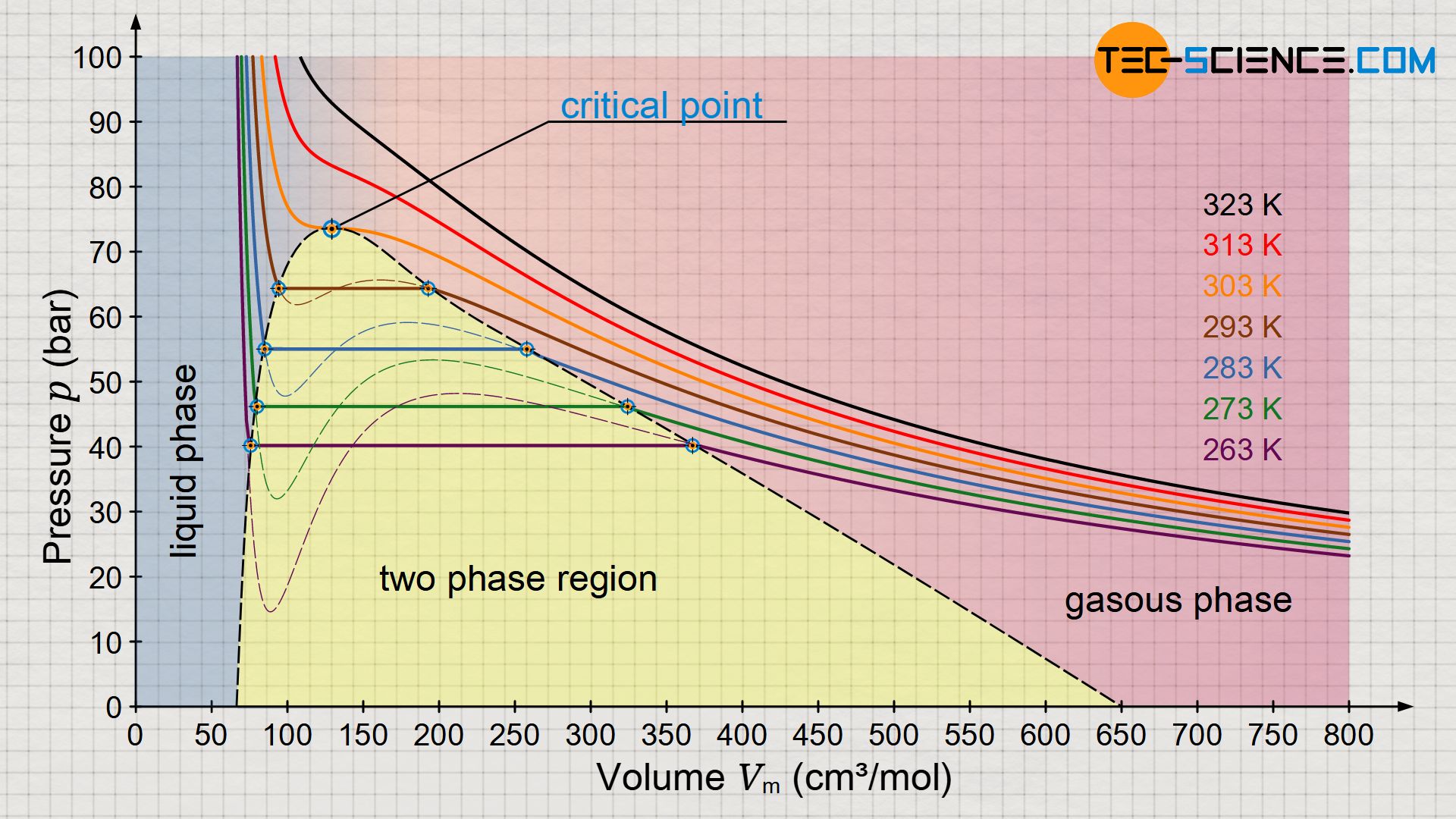
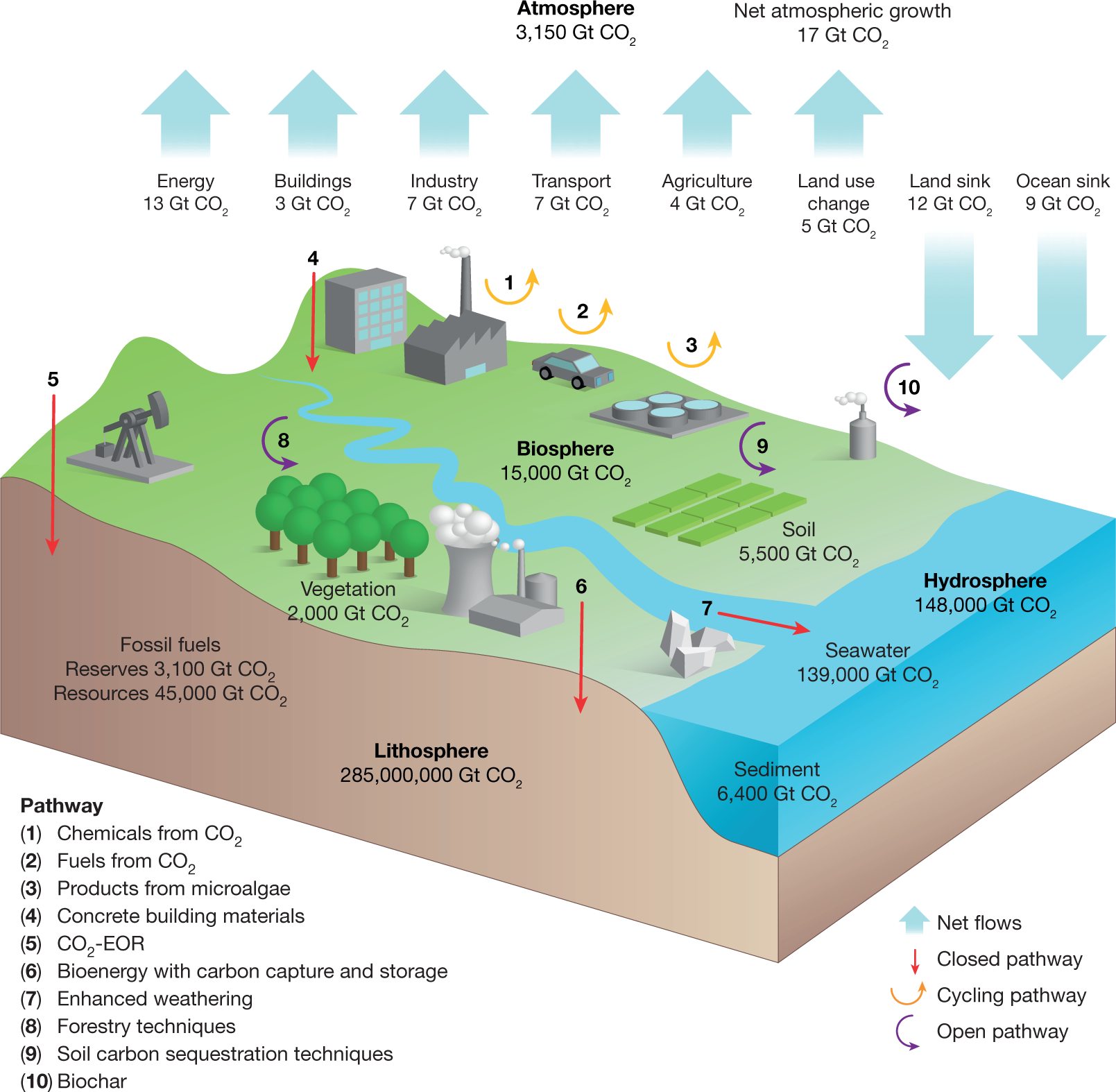
Experimental Study on the Density-Driven Carbon Dioxide Convective Diffusion in Formation Water at Reservoir Conditions | ACS Omega

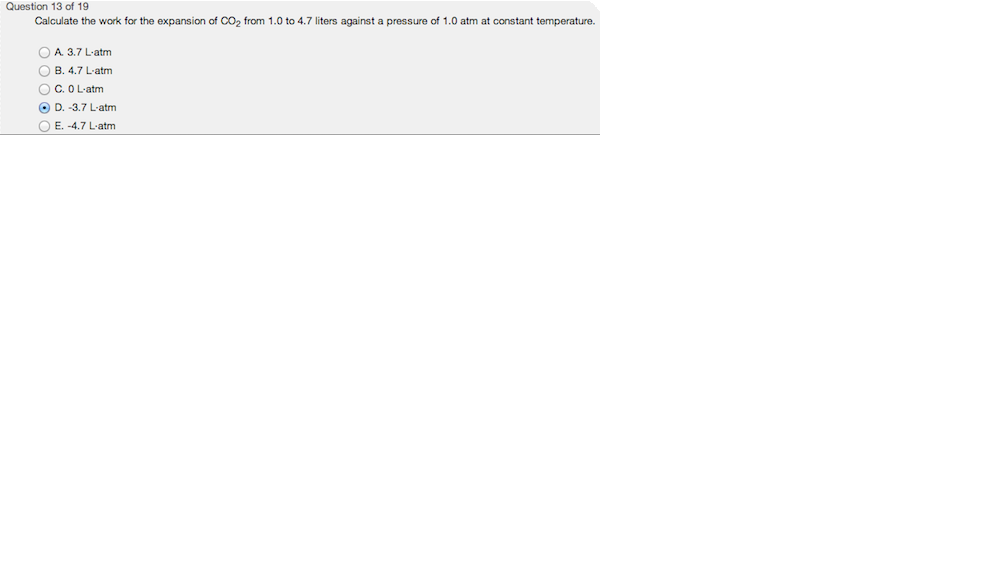
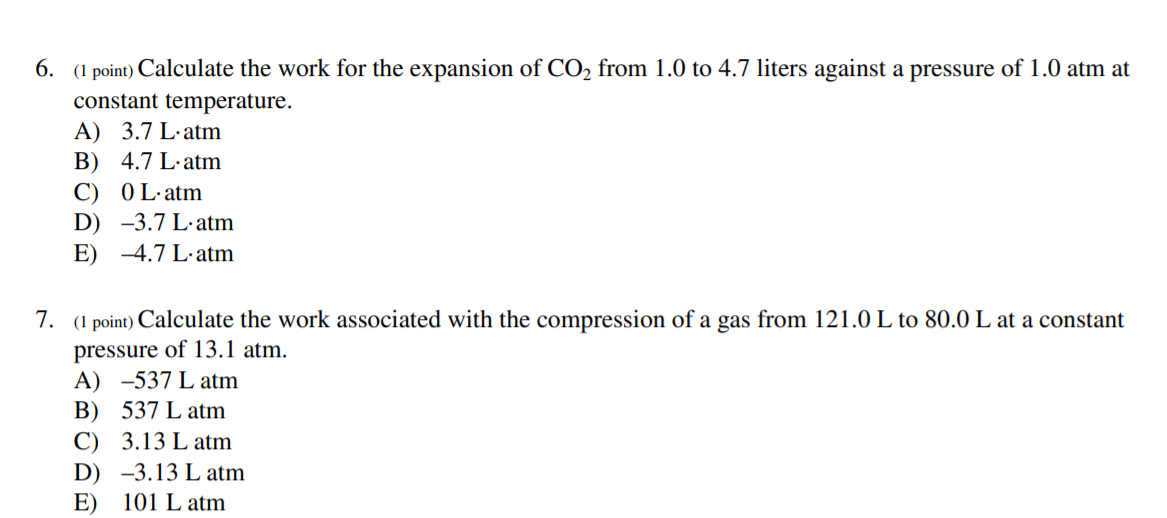
Chapter 2 Chem.docx - Ans: d Calculate the work for the expansion of CO2 from 1.0 to 5.8 liters against a pressure of 1.0 atm at constant | Course Hero

Chapter 2 Chem.docx - Ans: d Calculate the work for the expansion of CO2 from 1.0 to 5.8 liters against a pressure of 1.0 atm at constant | Course Hero

SOLVED: (10 pts) Calculate the maximum non-expansion work per mole that may be obtained from fuel cell in which the chemical reaction is the combustion of methane at 298 K. CHa(g) Oz(g)
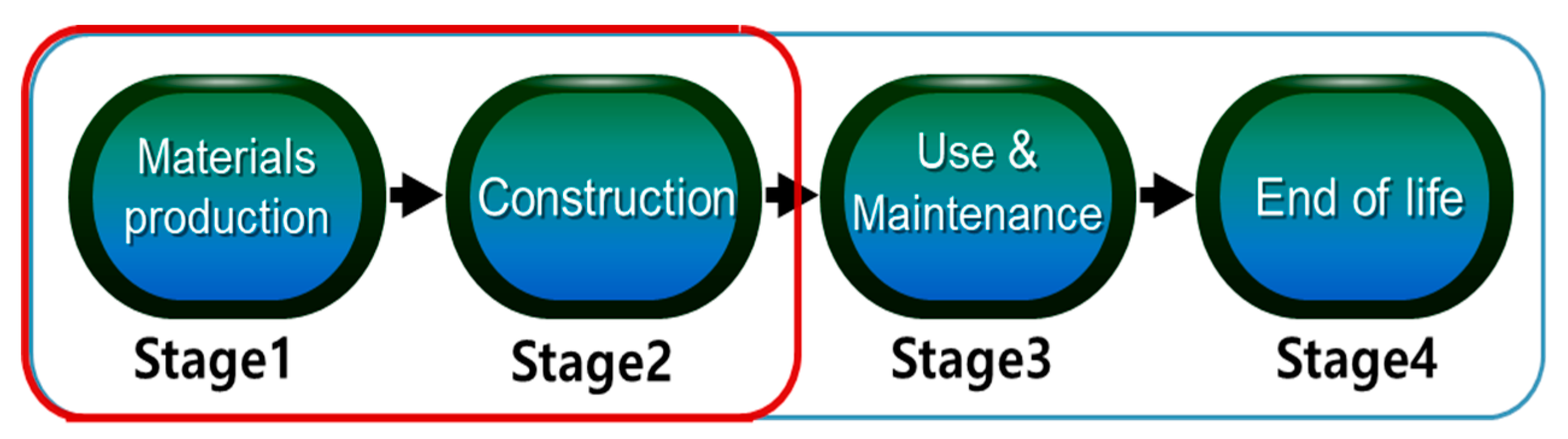
Applied Sciences | Free Full-Text | CO2 Emission Calculation Method during Construction Process for Developing BIM-Based Performance Evaluation System

Chapter 2 Chem.docx - Ans: d Calculate the work for the expansion of CO2 from 1.0 to 5.8 liters against a pressure of 1.0 atm at constant | Course Hero
14 g oxygen at 0^(@)C and 10 atm is subjected to reversible adiabatic expasnion to a pressure of 1atm. Calculate the work done in a. Litre atomsphere. b. Caloride (given, C(P)//C(V) =

Calculate maximum work At 303k ,22×10^-³ kg of co2 was expanded isothermally and reversibly from 11 2dm^3 to 16 8 - Chemistry - - 13575559 | Meritnation.com